The main benefit of using SAAs and their related nitrogen and carbon congeners in the production of peptidomimetics and glycomimetics is that their properties can be readily. Definition- a peptidomimetics is a small protein-like chain designed to mimic a peptide.

Figure 2 From Peptides And Peptidomimetics In Medicinal Chemistry Semantic Scholar
Self-structural organizations such as turns helices sheets and loops can be accessed by chemical modifications of amino acids or peptides.

. Thus the carbohydrate scaffold can display substituents to control distance. Combinatorial chemistry and High throughput screening. O either modifying the atoms involved in backbone formation of a peptide or in manipulating the side-chain moiety for example by introducing chemical tethers as rigidifying elements.
Synthesis of Acids and Amino Acids by Selective Oxidation of Primary. Access to novel amino acids as peptide isosteres has been pursued by. Recent advances in the use of solid-phase organic synthesis have paved the.
The design of even smaller peptidomimetics seems possible as an SAR study for class II reports nanomolar affinity of a N- and C-terminally capped tetrapeptide 20 including the unnatural amino acids cyclohexylalanine and norvaline at position 1 and 3 respectively Cunningham et al 1997. Peptidomimetics represent an important field in chemistry pharmacology and material science as they circumvent the limitations of traditional peptides used in therapy. To enable the rational design of new peptidomimetics with high bitter taste sensitivity we developed an in-house C program to map out amino acid preferences at different sequence positions based on the GA-PLS coefficients along with the scores of 615 amino acids.
A popular approach focused on proline-based bicyclic δ-amino. δ-Amino acids are isosteric replacements of dipeptide units and their application in the field of peptidomimetics has been extensively reported particularly for developing β-turn mimetics. Self-structural organizations such as turns helices sheets and loops can be accessed by chemical modifications of amino acids or peptides.
Although no drug molecule has been developed a number of novel potent ligands for receptors have been identified. A medicinal chemistry approach where parts of the peptide are successively replaced by non-peptide moieties until getting a non-peptide molecule and a biophysical approach where a hypothesis of the bioactive form of the peptide. Therapeutic values of Peptidomimetics design of peptidomimetics by manipulation of the amino acids modification of the peptide backbone incorporating conformational constraints locally or globally.
Among the analogs of compound 18 compound 32 a cyclic d-amino acid-containing peptidomimetic was found to have an IC 50 value in the nanomolar range in HER2-overexpressing cancer cell lines. For eg-anticancer peptidomimetics can bind to target proteins in order to induced cancer celles. The design and synthesis of peptidomimetics are most important because of the dominant position peptide and protein-protein interactions play in molecular recognition and signaling especially in living systems.
12 Hrs 5 Peptidomimetics Therapeutic values of Peptidomimetics design of peptidomimetics by manipulation of the amino acids modification of the peptide backbone incorporating conformational constraints locally or globally. Inspired by previous work concerning design of antibacterial helical β 3-peptides the group of Gellman explored α-peptideβ 3-peptide hybrids consisting of L-Lys and L-Leu as well as the constrained via five-membered rings β 3-amino acids ACPC and APC in a 11 ratio and with an alternating design also referred to as αβ-peptides. A common approach to dipeptide isosteres is the design and synthesis of conformationally biased δ-amino acids.
Recent developments in the solid phase synthesis of prospecting combinatorial libraries syntheses of pyranose based sugar amino acids SAAs and other scaffolding for peptidomimetics are included. 38 Further development of such noncanonical amino acids offers a. O Moreover peptidomimetic chemistry has been oriented to the development of higher isosteres taking.
Rational design of non-covalently and covalently binding enzyme inhibitors. Chemistry of prostaglandins leukotrienes and. 37 Amino acid variants with unsaturated side chains have also been described.
Up to 10 cash back Abstract. Exploring Ramachandran and Chi space. They form the basis of important classes of enzyme inhibitors they act as receptor agonists and antagonists and they have even been used to mimic DNA structure.
This review focuses on structural aspects of carbohydrates in peptide design. Rational design of non-covalently and covalently binding enzyme inhibitors. Sibylle et al 2001.
Basically there are two different approaches to design peptidomimetics. Chemistry of prostaglandins leukotrienes and. Protein design seeks to identify the properties of amino acid sequences that fold to predetermined structures with desirable structural and functional characteristics.
To improve the stability of the peptidomimetic d-amino acids were introduced into the peptidomimetic and several analogs of compound 18 were designed. Many SAAs have been exploited as templates and synthons in the design of peptidomimetics of biologically active peptides Frank et al 2002. They are typically arise from modification of an exsisting peptide or by designing similar systems that mimic peptides such as proteins and beta peptides.
Peptidomimetics have found wide application as bioavailable and often potent mimetics of natural peptides. Peptidomimetics represent an important field in chemistry pharmacology and material science as they circumvent the limitations of traditional peptides used in therapy. 12 Hrs 5 Peptidomimetics Therapeutic values of Peptidomimetics design of peptidomimetics by manipulation of the amino acids modification of the peptide backbone incorporating conformational constraints locally or globally.
The examples presented here emphasize the use of metathesis in several aspects of peptides and peptidomimetics from amino acid synthesis design and synthesis of secondary structure elements and. Therapeutic values of Peptidomimetics design of peptidomimetics by manipulation of the amino acids modification of the peptide backbone incorporating. Different techniques Solid phase synthesis.
Conformationally constrained amino acids and peptides in the design of bioactive polypeptide. Peptide drugs occupy a middle space between proteins and small molecules and it is hoped that they can target undruggable intracellular proteinprotein interactions. This compendium focuses on functionalised sugar amino acids SAAs and their 3- to 6-membered nitrogen heterocyclic and carbocyclic analogues.
Sugars with TCICATEMPO to produce SAAs could be achieved by careful manipulation of reaction conditions and. This study gives further support to a binding mode similar to. To obtain BTD peptidomimetics with higher predictive activity we alternatively selected the.
In most cases the carbohydrate will function as a scaffold ie as a relatively rigid structure to which amino acids amino acid mimics side-chain mimics peptides etc.

Computer Aided Design Of Amino Acid Based Therapeutics A Review Dddt
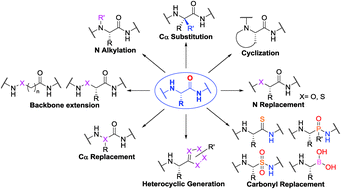
Peptidomimetics Via Modifications Of Amino Acids And Peptide Bonds Chemical Society Reviews Rsc Publishing

Solid Phase Synthesis Of Peptidomimetics With Peptide Backbone Modifications Abdildinova 2021 Asian Journal Of Organic Chemistry Wiley Online Library

Pdf Chemical Modifications Designed To Improve Peptide Stability Incorporation Of Non Natural Amino Acids Pseudo Peptide Bonds And Cyclization

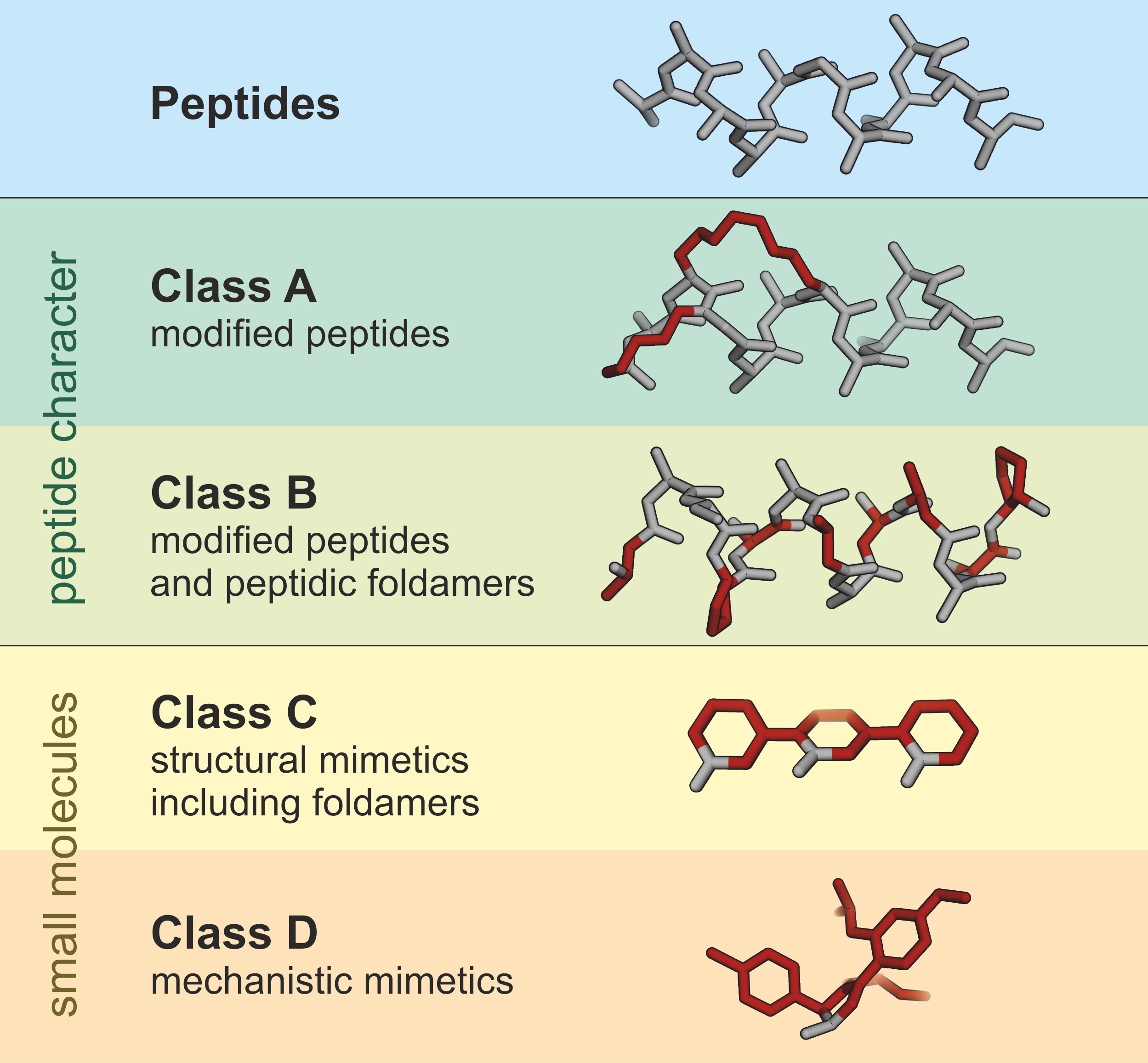


0 comments
Post a Comment